Chondrogenic differentiation: from stem cell to cartilage
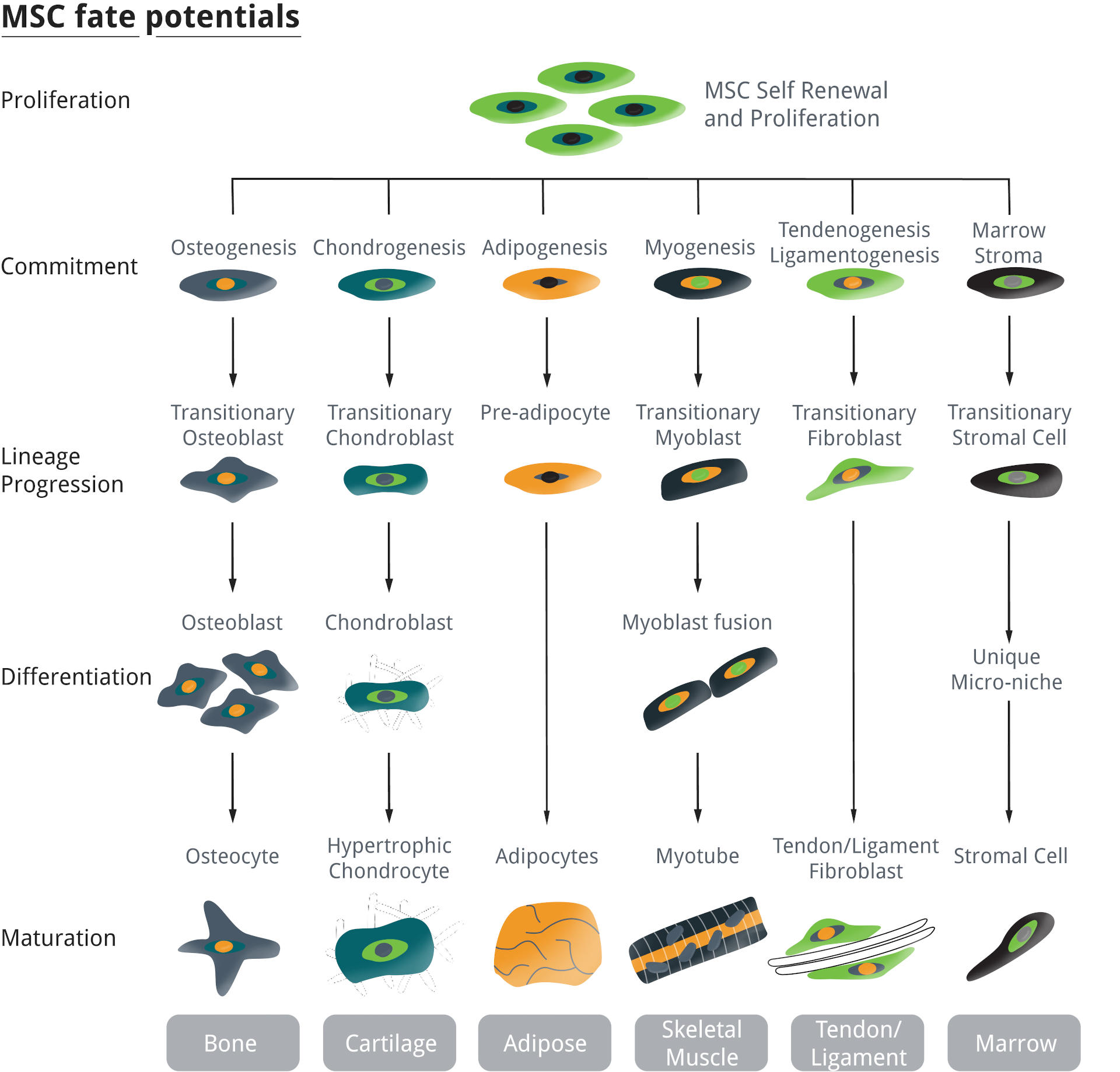
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a variety of cell types including osteoblasts (bone cells), neurones (nerve cells), chondrocytes (cartilage cells), myocytes (muscle cells), and adipocytes (fat cells). In this blogpost, we focus on chondrocytes, the cells that produce the cartilage that fills our joint and protects our bones. Differentiation is induced by specific culture conditions and defined media containing transforming growth factor β (1). Two types of cell are formed during chondrogenesis: chondroblasts, and chondrocytes (Fig. 1). Chondroblasts are progenitor cells that secrete the extracellular matrix (ECM), while chondrocytes are involved in nutrient diffusion and matrix repair. Both cell types are required to form cartilage.
Observe chondrogenic differentiation in real time using Nanolive cell imaging
Here, we capture the early stages of chondrogenesis live, and in real time, using Nanolive cell imaging. We induced chondrogenic differentiation using Promocell’s MSC chondrogenic differentiation medium and imaged how cells respond 7, 9 and 10 days after medium addition. Images were captured every 30 mins, using the 10×10 grid-scan mode (an area equivalent to 900 µm) on Nanolive’s CX-A.
Figure 1
Mesenchymal stem cells (MSCs) are multipotent stromal cells capable of self-renewal and proliferation or differentiation into a variety of cell types, including osteoblasts (bone cells), chondrocytes (cartilage cells), adipocytes (fat cells), myocytes (muscle cells), tenocytes (tendon/ligament cells) and adipocytes (fat cells). Schema adapted from https://discovery.lifemapsc.com/
Chondroblast cells contain highly organized intercellular F-actin fiber networks
At day 7, we observe a highly confluent population of chondroblast cells. One of the most striking features of these cells are the numerous intercellular F-actin fibers they contain in their cytoplasm. Fibers are distributed throughout the cytoplasm in a highly organized structural network that extends from the nuclear membrane to the periphery of the cell. The white globular structures in the cytoplasm are very likely collagen type II granules (3). This collagen will eventually be secreted to participate to the formation of the extracellular matrix of the cartilage. Black vacuoles are also present in the cytoplasm. These vacuoles are part of the endocytic pathway that the cell uses to degrade and recycle old cellular components.
Collagen accumulates during differentiation while vacuoles appear
As differentiation progresses, the amount of collagen and the number of vacuoles increases. The distribution of collagen granules reflects the position of the endoplasmic reticulum (ER), where collagen is massively produced.
The first step towards chondrogenic tissue structure?
By day 10, cells start to separate leaving small gaps between them. When fully differentiated, chondrocytes form chondrogenic centers surrounded by cavities in the matrix called lacunae. It is possible that the gaps we observe between cells is the first step towards cells assuming the structure of chondrogenic tissue, although this would need to be confirmed by longer experiments.
Figure 2
Collagen granules (white globular structures) accumulate and vacuoles (black structures) appear during chondrogenic differentiation. Here we observe the differences: A) 7 days; B) 9 days and C) 10 days post differentiation media addition. Note the numerous intercellular F-actin fibers present in the cell cytoplasm.
References
(1) Seo S, Na K. Mesenchymal stem cell-based tissue engineering for chondrogenesis. Journal of Biomedicine and Biotechnology. 2011 Jan 1;2011.
(2) Solchaga, L.A., Penick, K.J. and Welter, J.F., 2011. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. In Mesenchymal stem cell assays and applications (pp. 253-278). Humana Press.
(3) Mackay, A.M., Beck, S.C., Murphy, J.M., Barry, F.P., Chichester, C.O. and Pittenger, M.F., 1998. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue engineering, 4(4), 415-428.
Read our latest news
Cytotoxic Drug Development Application Note
Discover how Nanolive’s LIVE Cytotoxicity Assay transforms cytotoxic drug development through high-resolution, label-free quantification of cell health and death. Our application note explores how this advanced technology enables real-time monitoring of cell death...
Investigative Toxicology Application Note
Our groundbreaking approach offers a label-free, high-content imaging solution that transforms the way cellular health, death, and phenotypic responses are monitored and quantified. Unlike traditional cytotoxicity assays, Nanolive’s technology bypasses the limitations...
Phenotypic Cell Health and Stress Application Note
Discover the advanced capabilities of Nanolive’s LIVE Cytotoxicity Assay in an application note. This document presents a detailed exploration of how our innovative, label-free technology enables researchers to monitor phenotypic changes and detect cell stress...
Nanolive microscopes
Swiss high-precision microscopes that look instantly inside label-free living cells in 3D

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging