Transcription factors: a promising therapeutic target for anti-cancer treatment
Cancer is a disease characterized by aberrant gene expression. Transcription factors (the regulatory proteins that bind to specific DNA sequences controlling gene expression) are thus, promising therapeutic targets for anti-cancer treatments. Many transcription inhibitors already exist: α-amanitin, actinomycin D, DRB, flavopiridol and triptolide, to name but a few (1). Some (e.g., actinomycin D) have been used in chemotherapy for a long time (2), while others (e.g., triptolide) were identified more recently (3).
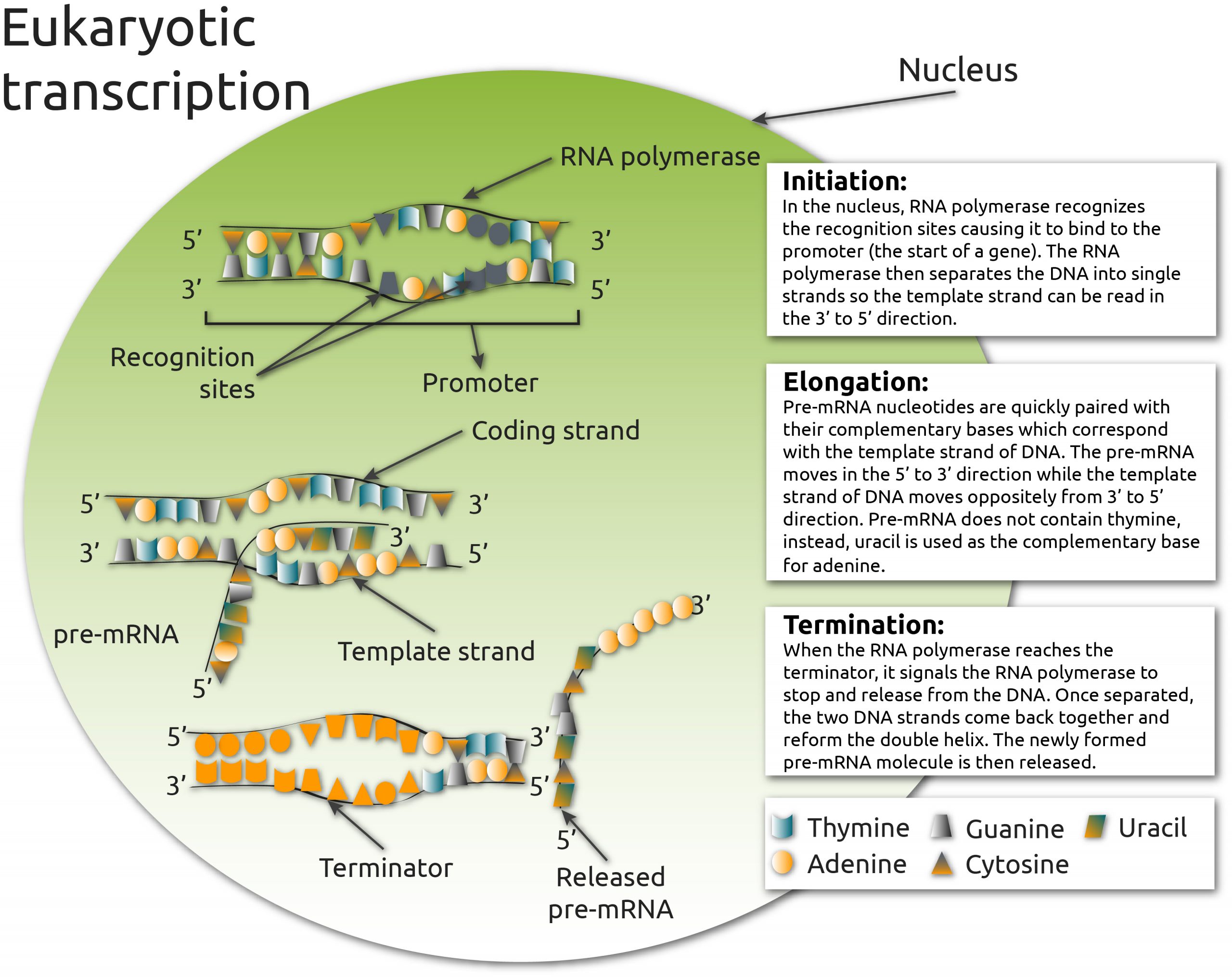
Transcription can be broadly divided into three stages: initiation, elongation, and termination (Fig. 1). The drugs named above target different parts of the transcription process. In this blogpost, we focus on actinomycin D; a DNA intercalator that inhibits RNA polymerase elongation, and DRB; a kinase inhibitor of RNAP II elongation (1).
Fig. 1. The three phases of eukaryotic transcription: initiation, elongation, and termination.
In the initiation phase, RNA polymerase binds to the promoter region of a gene and separates the DNA into a single strand that can be read in the 3′ to 5′ direction. This strand is used as a template for pre-mRNA nucleotides, which are added in the 5’ to 3’ direction during the elongation phase. When RNA polymerase reaches the terminator, it releases the DNA. The two DNA strands reform the double helix and the newly-formed pre-mRNA molecule is then released.
Changes in nuclear structure offer a means of testing the efficiency of transcription inhibitors
The nucleus and its nucleoli are highly dynamic organelles that undergo continuous morphological re-modelling (4). Transcription inhibition results in major changes in nuclear structures particularly in the nucleoli (4). Changes in the shape, size, and number of nucleoli per nucleus are excellent markers of cell stress or disease (5). Detecting nuclear changes has, to date, usually involved bespoke protein, DNA, or RNA labels, or high-throughput DNA sequencing (6). Some studies have used imaging, but are normally end-point techniques e.g., DNA staining (7), which are time-consuming and cannot capture dynamic information.
Nanolive cell imaging offers a unique label-free solution to detecting nuclear changes based on holotomography, which measures the refractive index (RI) of living cells (8). Because different organelles have different densities, and thus RI signatures (9), we can visualize the nucleus, the nuclear membrane, and nucleoli without the addition – and limitations of – fluorescent labels.
Nanolive imaging detects major changes in the nucleus, nucleoli and nucleoplasm following transcription inhibition
Here, 3T3-derived pre-adipocyte cells were grown into DMEM and exposed to either 20 µM actinomycin D or 25 µM DRB. Cells were imaged once every 6 secs for 15 mins, and 1 h respectively, using the 3D Cell Explorer-fluo. Both inhibitors caused major changes in nuclear structures, particularly the nucleoli, but the responses were not identical.
Actinomycin D caused major changes in all the nuclear structures. The nucleoli shrank/disintegrated, the nucleus swelled in size and the nucleoplasm became more granular over time, changes consistent with the segregation of the fibrillar center, dense fibrillar center and granular components of the nucleolus, reported by other studies (5). The change in nuclear structure can be detected by a cell metric called granularity; a texture measurement that describes how the RI signal is distributed at a certain scale (here: 5 pixels). A change in granularity, here a sharp increase over the first 30 mins followed by a moderate decrease over the next 30 mins, is indicative of a re-distribution of the organelles of the cell.
DRB seemingly had less profound nuclear impacts (at least at this concentration), but interestingly, the cell did develop numerous endocytic vacuoles (black structures) in its cytoplasm; a sign of cellular stress.
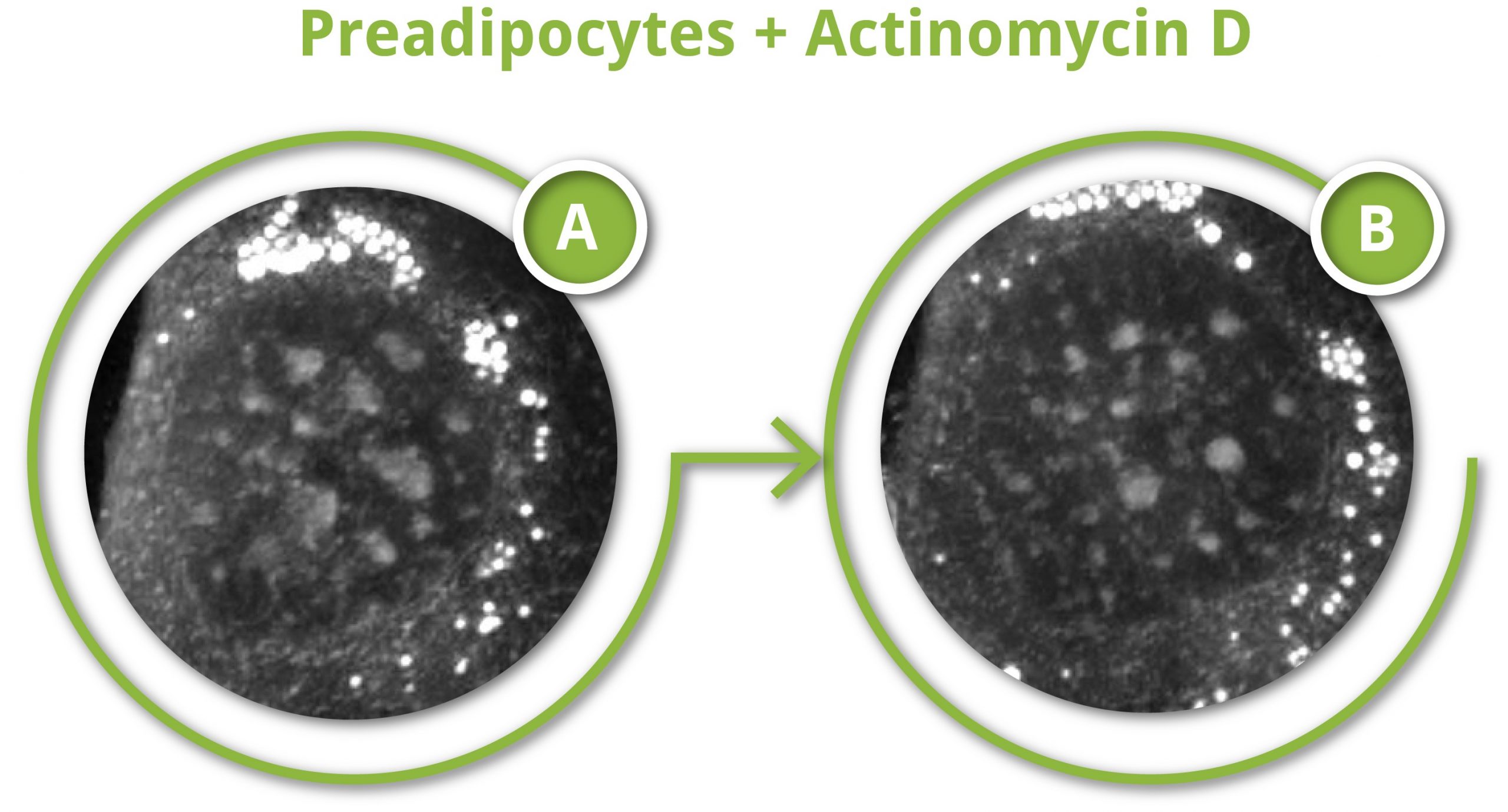
3T3-derived pre-adipocyte cells grown into DMEM and exposed to either 20 µM actinomycin D (right and figure) or 25 µM DRB (left)
Time resolved changes in granularity following 20 µM actinomycin D exposure (in figure: A = initial time, B = final time). DRB seemingly had less profound nuclear impacts (at least at this concentration), but interestingly, the cell did develop numerous endocytic vacuoles (black structures) in its cytoplasm; a sign of cellular stress.
How can Nanolive imaging be used in future nuclear studies?
This study demonstrate that Nanolive imaging can detect nuclear structures with high precision, but it is difficult to assess how much of an effect these drugs have had, since so little is known about the variation that exists in nuclear morphology in unperturbed conditions. Nuclear resolution is maintained in our automated microscope, the CX-A, which would make it easy to scale up single-cell observations to the population level. Determining the baseline variation that exists in the nuclear metrics (e.g. size of the nucleus relative to the size of the cell; average number of nuclei per nucleus, average size and shape of the nuclei) of unperturbed cells would be a good starting point for any study.
Once the natural variation in nuclear morphology is understood, drugs of interest can be applied, and nuclear changes can be compared to unperturbed conditions. This will allow to identify specific nuclear and/or nucleolar phenotypes and their role in disease. Alternatively, a screening approach could be taken, whereby multiple drugs are tested at the same time and cells are screened for changes in nuclear morphology, which could help identify novel inhibitors of transcription.
References
(1) Bensaude O. Inhibiting eukaryotic transcription. Which compound to choose? How to evaluate its activity? Which compound to choose? How to evaluate its activity?. Transcription. 2011 May 1;2(3):103-8.
(2) Rahman YE, Cerny EA, Tollaksen SL, Wright BJ, Nance SL, Thomson JF. Liposome-encapsulated actinomycin D: potential in cancer chemotherapy. Proceedings of the Society for Experimental Biology and Medicine. 1974 Sep;146(4):1173-6.
(3) Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Molecular cancer therapeutics. 2003 Jan 1;2(1):65-72.
(4) Lafontaine DL, Riback JA, Bascetin R, Brangwynne CP. The nucleolus as a multiphase liquid condensate. Nature Reviews Molecular Cell Biology. 2020 Sep 1:1-8.
(5) Boulon, S., Westman, B. J., Hutten, S., Boisvert, F. M. & Lamond, A. I. The nucleolus under stress. Mol. Cell 40, 216–227 (2010).
(6) Yamamoto N, Jiang P, Yang M, Xu M, Yamauchi K, Tsuchiya H, Tomita K, Wahl GM, Moossa AR, Hoffman RM. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Research. 2004 Jun 15;64(12):4251-6.
(7) Pagliara S, Franze K, McClain CR, Wylde GW, Fisher CL, Franklin RJ, Kabla AJ, Keyser UF, Chalut KJ. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nature materials. 2014 Jun;13(6):638-44.
(8) Cotte Y, Toy F, Jourdain P, Pavillon N, Boss D, Magistretti P, Marquet P, Depeursinge C. Marker-free phase nanoscopy. Nature Photonics. 2013 Feb;7(2):113-7.
(9) Sandoz PA, Tremblay C, van der Goot FG, Frechin M. Image-based analysis of living mammalian cells using label-free 3D refractive index maps reveals new organelle dynamics and dry mass flux. PLoS biology. 2019 Dec 19;17(12):e3000553.
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations