Toxoplasmosis: public enemy number 1 of pregnant women
Toxoplasmosis – infection arising from the obligate intracellular parasite Toxoplasma gondii – is one of the most common diseases in the world. The World Health Organization (WHO) estimates that more than 2 million people a year contract toxoplasmosis in Europe alone (1), and that globally, over one third of the population is infected at some point in their lives (2). Although some people suffer flu-like symptoms, most cases are asymptomatic. One group of the population though are at a much higher risk: pregnant women.
A recent global meta-study of serologic tests revealed that out of 723,655 pregnant women, 1.9 % (13,749) were infected with T. gondii (3). These values differed significantly between countries and regions, with women from developing countries such as Yemen facing the highest risk (3). The consequences of contracting Toxoplasmosis; miscarriage, stillbirth, foetal death, neurocognitive deficits, and child disability (4), can be devastating.
So how can pregnant women protect themselves from Toxoplasmosis? To answer this, one must first understand the complicated life cycle of T. gondii.
Intermediate hosts with asexual phases and definitive hosts with sexual phases: the T. gondii life cycle has it all!
The lifecycle of T. gondii contains both sexual and asexual stages, which occur in different host species (Fig. 1). The sexual stage only occurs in cats (felids, wild or domestic) making them the definitive hosts, while the asexual stage can occur in virtually all warm-blooded animals, making them intermediate hosts (Fig. 1). Why cats? Well, our feline friends lack an enzyme called delta-6-desaturase (D6D) in their intestine, which normally breaks down linoleic acid, which turns out to be, you got it, an essential component of T. gondii sexual reproduction (5).
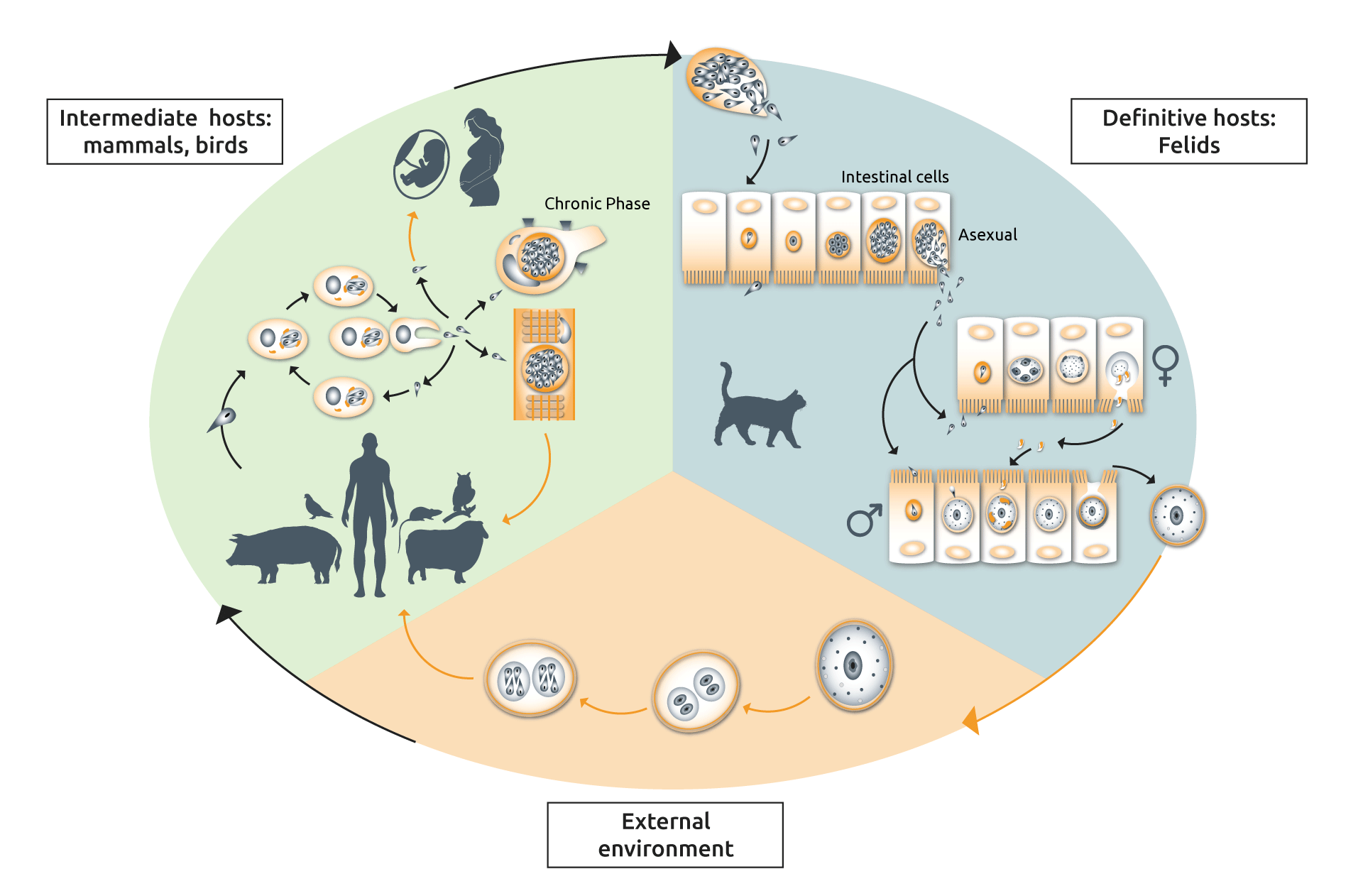
Fig. 1
The complicated life cycle of Toxoplasma gondii. Diagram adapted from (6,7).
So, story time. Imagine a mouse ingests T. gondii oocysts. The oocysts are transported to the mouse’s stomach where the thick cell wall of the oocyst is broken down, releasing the parasites inside. Parasites invade its hosts epithelial lining before differentiating into tachyzoites (a motile and quick division phase), and then bradyzoites (slower division within tissue cysts). Within 7 to 10 days, fully formed tissue cysts are present in the brain, which induces a behavioural phenomenon called ‘fatal attraction’ whereby the mouse loses its aversion to cat odours (8).
Now imagine a pet cat, out roaming his territory at night, bumps into the dozy mouse. Delighted with his prowess he kills the mouse with one swift bite to the head, loads it in his mouth and trots back to his house to “offer” the mouse to his owner. Little does he know that that he is carrying more than just the mouse; that small blow was enough for some tissue cysts to enter the cat’s digestive system.
The bradyzoites are freed upon the dissolution of the cyst wall in the cat’s stomach, and immediately invade the epithelial cells lining the cat’s intestine. Here, they differentiate into five morphologically distinct types of cells called schizonts. Schizonts become merozoites before developing into the macrogametes and microgametes required to produce diploid oocysts. These oocysts then find their way back into the environment via the usual route. An infected cat excretes between 2 and 20 million oocysts per day in their faeces, for up to 10 days post infection (9, 10).
Once in the external environment, oocysts undergo sporulation; a maturation process to produce eight haploid sporozoites encased in an oocyst wall (Fig. 1). At this point the oocysts are infectious and can be eaten by a new mouse. Brings a whole new perspective to Tom and Jerry, huh?
Ok, but what about the link between humans and T. gondii? How can I avoid becoming infected?
In humans, infection normally occurs through the consumption of raw or undercooked meat (i.e., through eating an intermediate host). However, contamination can also occur through indirect contact with cat faeces including infected cat litter or soil. For both, prevention is better than cure. Common guidelines include wearing gloves while gardening; never eating raw or undercooked meat; washing all fruits and vegetables before eating them; avoiding unpasteurized milk; and (for the cat lovers) wear gloves and a face mask when changing the litter box.
Of course, the adage applies: prevention requires awareness and education, which is why we are happy to share this remarkable video of Toxoplasma parasites inside mammalian fibroblasts taken using Nanolive’s unique label-free 3D Cell Explorer-fluo microscope. Images were taken every 20 secs for 1 h by Dr. Karine Frénal from the MFP laboratory in Bordeaux, France.
“It was a real surprise for me to see so well the parasites and the host cells” Dr. Karine Frénal
High-resolution time-lapse imaging of T. gondii-infection dynamics in living cells
The video above features an infected fibroblast cell containing six separate parasite-filled, membrane-bound parasitophorous vacuoles in its cytosol. Pay attention to the furthest right compartment. At the beginning of the video the compartment contains four parasites. At the 10 min mark parasites undergo division and shortly after they can be observed rotating and re-arranging their structure. Capturing such dynamic behaviour is only possible thanks to the high spatio-temporal resolution of label-free imaging.
This is not the first time Nanolive imaging has been used to investigate the host-pathogen interactions involved in T. gondii infection. That honour goes to Dr. Zahady Velásquez from the Justus-Liebig University in Gießen, Germany, who used Nanolive imaging to calculate the proportion of mitotic cells able to complete cytokinesis in T. gondii-infected and non-infected cells. Through her research, Dr. Velásquez was able to show that T. gondii tachyzoite infection leads to increased host cell proliferation and a greater number of multi-nucleated host cells. Read the publication here.
References
[1] https://www.euro.who.int/en/health-topics/disease-prevention/food-safety/news/news/2016/11/toxoplasmosis-greater-awareness-needed (website visited 19.10.2021).
[2] Montoya, JG. and Liesenfeld, O. Toxoplasmosis. Lancet 363: 1965-1976 (2004).
[3] Bigna, JJ. et al. Sci. Rep. 21;10(1): 12102 (2020).
[4] Jones, JL. et al. Obstet. Gynecol. Surv. 56: 296-305 (2001).
[5] Martorelli Di Genova, B. et al. PLoS Biol. 20; 17(8): e3000364 (2019).
[6] Robert-Gangneux, F. and Dardé, ML. Clin. Microbiol. Rev. 25(2): 264-296 (2012).
[7] Ferguson, DJ. Memorias do Instituto Oswaldo Cruz. 104: 133-148 (2009).
[8] Berdoy, M. et al. Proc. Royal Soc. B. 7; 267(1452): 1591-1594 (2000).
[9] Dabritz, HA and Conrad, PA. Zoonoses Public Health. 57: 34–52 (2010).
[10] Martins, TA. Vet Parasitol. 249: 17-20 (2018).
Read our latest news
Cytotoxic Drug Development Application Note
Discover how Nanolive’s LIVE Cytotoxicity Assay transforms cytotoxic drug development through high-resolution, label-free quantification of cell health and death. Our application note explores how this advanced technology enables real-time monitoring of cell death...
Investigative Toxicology Application Note
Our groundbreaking approach offers a label-free, high-content imaging solution that transforms the way cellular health, death, and phenotypic responses are monitored and quantified. Unlike traditional cytotoxicity assays, Nanolive’s technology bypasses the limitations...
Phenotypic Cell Health and Stress Application Note
Discover the advanced capabilities of Nanolive’s LIVE Cytotoxicity Assay in an application note. This document presents a detailed exploration of how our innovative, label-free technology enables researchers to monitor phenotypic changes and detect cell stress...
Nanolive microscopes

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging



