Background
Chondrogenic differentiation describes how cartilage is formed from mesenchymal stem cells (MSCs). Cells pass two morphological stages; first they differentiate into chondroblasts, which then mature into chondrocytes. Chondroblasts are responsible for the secretion of the extracellular matrix (ECM), while chondrocytes are involved in nutrient diffusion and matrix repair. Here, we show it is possible to image the early morphological changes cells undergo before they assume the structure of chondrogenic tissue while using a completely label-free approach on live cells.
Experimental approach
Mesenchymal stem cells (MSCs) were grown on fibronectin-coated dishes until 100% confluency was reached. Promocell’s MSC chondrogenic differentiation medium was added to induce differentiation. Cells were imaged 7, 9 and 10 days after medium addition. One image was captured every 30 mins, using the 10×10 grid-scan mode (area = 900 μm) on Nanolive’s automated microscope, the CX-A. The resulting images were subjected to a maximum intensity projection (MIP) along the Z-axis and then stitched to produce videos.
Observations
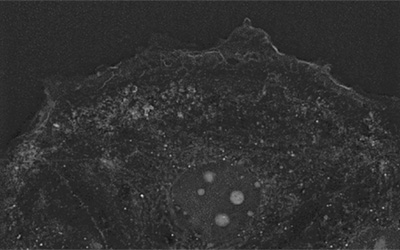
• Nanolive’s CX-A was able to capture the striking sub-cellular features of individual chondroblast cells; collagen granules (A, white structures), F-actin fibers (B), and endocytic vacuoles (C, black structures), in exceptional detail.
• The high-spatial resolution of the images makes it possible to investigate how structures are distributed inside the cell. Collagen granules predictably reflect the position of the endoplasmic reticulum (ER), where they are produced (A). F-actin fibers are highly organized, extending from the nuclear membrane to the periphery of the cell (B). Endocytic vacuoles tend to cluster around the cell’s nucleus (C).
• The temporal aspect of this experiment (cells imaged at 7, 9 and 10 days) revealed cells accumulate collagen and endocytic vacuoles as differentiation progresses.
• This study proves it is possible to capture the early stages of chondrogenesis live, and in real time, with Nanolive cell imaging. Future studies could attempt to inhibit collagen synthesis or perturb endocytic pathways, to better understand the role these processes play in differentiation.
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations