Cytokinesis, as we briefly introduced in our mitosis in Mesenchymal Stem Cells post, refers to the obtention of two separated daughter cells after mitosis[1]. The process of separation can coincide in time with anaphase and/or telophase, the two final steps in mitosis[2].
Human Mesenchymal Stem Cells from bone marrow (kindly provided by PromoCell GmbH) were cultured in low-serum cell growth medium and observed with the 3D Cell Explorer. Nanolive imaging allows for long-term (up to weeks) live cell imaging. Its low power laser is ideal for the visualization of mesenchymal stem cells, which are particularly sensitive to stresses such as phototoxicity due to the various excitation lights used in fluorescent imaging approaches.
Cytokinesis has classically been segmented based on time and morphology in four subprocesses. In the footage, the progression of cytokinesis is observed.
Microtubular organization
The cytoskeleton, membrane systems, and cell cycle engine work in a coordinated way and in harmony with numerous proteins throughout the process of cytokinesis[3], [4]. Even if the general mechanism is preserved, there are subtle variations among the different cell types in response to specific biological requirements (e.g.: cytokinesis in blastomeres vs. cytokinesis in stem cells)[5].
Anaphase and cytokinesis can happen at the same time and are orchestrated by key cytoskeletal structures called microtubule spindles. While anaphase relies on spindles to bring sister chromatids from the metaphase plate to the opposite ends of the cell, cytokinesis relies on central spindle to guide equatorial constriction[6],[7]. The position of the central spindles also helps to keep the two populations of sister chromatids correctly segregated, which prevents chromosome loss [8].
This footage permits the characterization of microtubule spindles as their high protein content has a detectable refractive index. According to literature, it is very likely that the central spindles could be found in the signaled position in Figure 1.
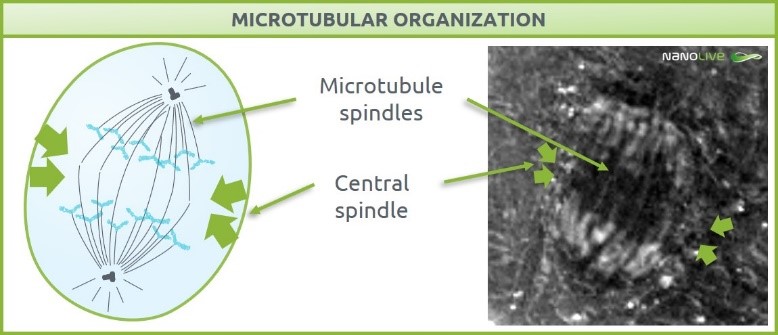
Figure 1. Microtubular organization. Microtubular spindles characterization and central spindle predicted localization.
Cleavage plane specification and contractile ring assembly
Cleavage takes place perpendicular to the axis of chromosome segregation. The mechanism underlying this cleavage plane specification is still unresolved [9]. Actin and myosin II are two cytoskeletal proteins essential in the formation of the contractile ring that will grow inward until the separation in two daughter cells [10], [11].
In the footage, we can predict the cleavage plane specification axis by drawing the axis of chromosome segregation (Figure 2, top). The effects of ring contraction are also visible (Figure 2, bottom). As we just described, the contraction results from the combination of force generation from the cytoskeletal structures helped by an increase of plasma membrane surface area, coming from membrane storage in vesicles and plasma membrane recycling [9]–[12].
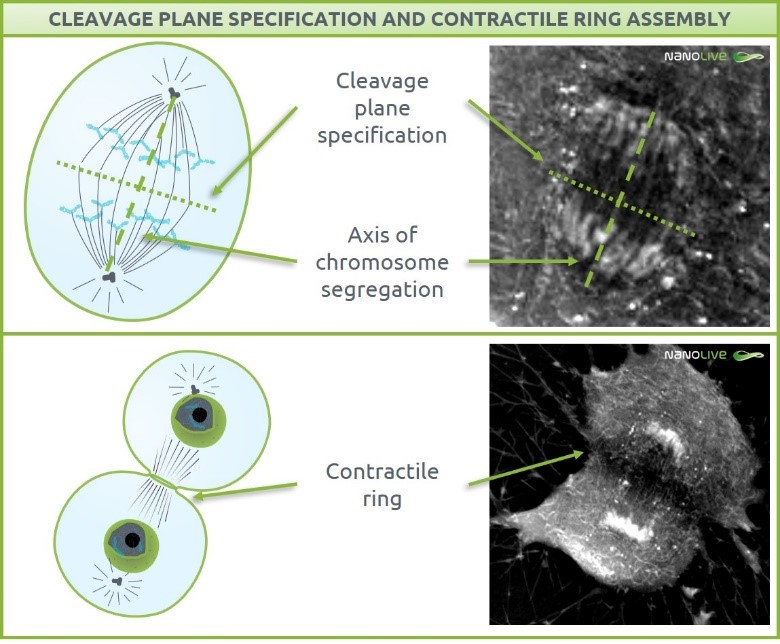
Figure 2. Cleavage plane specification (top) and contractile ring assembly effects (bottom)
Completion
The final and optional step in cytokinesis is completion. Two independent daughter cells are obtained as a result of completion. Even if it usually takes place, there are some exceptions. In specific cell types such as in embryotic blastomeres, the cells remain connected via intercellular bridges instead of undergoing a final scission [9]. Nevertheless, completion is important as a mechanism to prevent aneuploid cell accumulation [13].
After ring contraction, the two daughter cells are connected by the previously mentioned midbody structure (Figure 3, top). This bridge-shaped structure is formed with tightly bundled, antiparallel microtubules deriving from the central spindle and it gets narrower as cytokinesis progresses. The high content of proteins in the spindles and their refractive index make them detectable under the 3D Cell Explorer. Removal of the cytoskeletal components of the midbody and membrane fission leads to abscission of the two daughter cells (Figure 3, bottom) [14], [15]. Centrosomes seem to also play a role in completion, as they had been localized in the intercellular bridge late in cytokinesis [16].
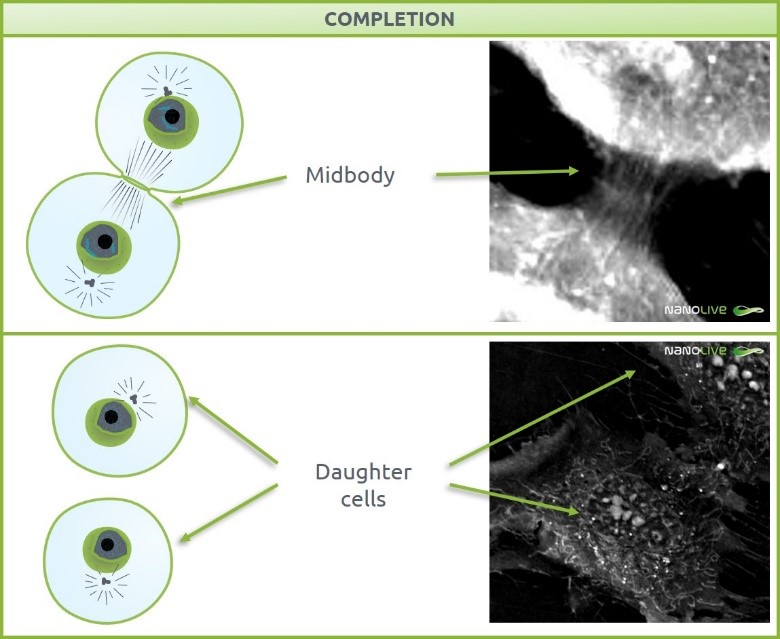
Figure 3. Completion. Top: Midbody structure. Bottom: Daughter cells after abscission.
[1] T. E. Schroeder, “The contractile ring,” Zeitschrift für Zellforsch. und Mikroskopische Anat., vol. 109, no. 4, pp. 431–449, 1970.
[2] M. Mishima, V. Pavicic, U. Grüneberg, E. A. Nigg, and M. Glotzer, “Cell cycle regulation of central spindle assembly,” Nature, vol. 430, no. 7002, pp. 908–913, Aug. 2004.
[3] “MitoSys: Systems Biology of Mitosis.” [Online]. Available: http://www.mitosys.org/.
[4] “MitoCheck.” [Online]. Available: https://www.mitocheck.org/downloads.shtml.
[5] T. Q. P. Uyeda, A. Nagasaki, and S. Yumura, “Multiple Parallelisms in Animal Cytokinesis,” in International review of cytology, vol. 240, 2004, pp. 377–432.
[6] C. Mollinari et al., “Ablation of PRC1 by Small Interfering RNA Demonstrates that Cytokinetic Abscission Requires a Central Spindle Bundle in Mammalian Cells, whereas Completion of Furrowing Does Not,” Mol. Biol. Cell, vol. 16, no. 3, pp. 1043–1055, Mar. 2005.
[7] Y. H. Inoue, M. S. Savoian, T. Suzuki, E. Máthé, M.-T. Yamamoto, and D. M. Glover, “Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression,” J. Cell Biol., vol. 166, no. 1, pp. 49–60, Jul. 2004.
[8] A. F. Straight et al., “Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor.,” Science, vol. 299, no. 5613, pp. 1743–7, Mar. 2003.
[9] U. S. Eggert, T. J. Mitchison, and C. M. Field, “Animal Cytokinesis: From Parts List to Mechanisms,” Annu. Rev. Biochem., vol. 75, no. 1, pp. 543–566, Jun. 2006.
[10] S. Yumura, “Myosin II dynamics and cortical flow during contractile ring formation in Dictyostelium cells.,” J. Cell Biol., vol. 154, no. 1, pp. 137–46, Jul. 2001.
[11] K. Murthy and P. Wadsworth, “Myosin-II-Dependent Localization and Dynamics of F-Actin during Cytokinesis,” Curr. Biol., vol. 15, no. 8, pp. 724–731, Apr. 2005.
[12] J. G. Bluemink and S. W. de Laat, “NEW MEMBRANE FORMATION DURING CYTOKINESIS IN NORMAL AND CYTOCHALASIN B-TREATED EGGS OF XENOPUS LAEVIS: I. Electron Microscope Observations,” J. Cell Biol., vol. 59, no. 1, pp. 89–108, Oct. 1973.
[13] Q. Shi and R. W. King, “Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines,” Nature, vol. 437, no. 7061, pp. 1038–1042, Oct. 2005.
[14] A. Echard, G. R. X. Hickson, E. Foley, and P. H. O’Farrell, “Terminal Cytokinesis Events Uncovered after an RNAi Screen,” Curr. Biol., vol. 14, no. 18, pp. 1685–1693, Sep. 2004.
[15] L. H. Hartwell, T. A. Weinert, U. Euteneuer, and M. Bornens, “Checkpoints: controls that ensure the order of cell cycle events.,” Science, vol. 246, no. 4930, pp. 629–34, Nov. 1989.
[16] M. Piel, J. Nordberg, U. Euteneuer, and M. Bornens, “Centrosome-dependent exit of cytokinesis in animal cells.,” Science, vol. 291, no. 5508, pp. 1550–3, Feb. 2001.
Read our latest news
Cytotoxic Drug Development Application Note
Discover how Nanolive’s LIVE Cytotoxicity Assay transforms cytotoxic drug development through high-resolution, label-free quantification of cell health and death. Our application note explores how this advanced technology enables real-time monitoring of cell death...
Investigative Toxicology Application Note
Our groundbreaking approach offers a label-free, high-content imaging solution that transforms the way cellular health, death, and phenotypic responses are monitored and quantified. Unlike traditional cytotoxicity assays, Nanolive’s technology bypasses the limitations...
Phenotypic Cell Health and Stress Application Note
Discover the advanced capabilities of Nanolive’s LIVE Cytotoxicity Assay in an application note. This document presents a detailed exploration of how our innovative, label-free technology enables researchers to monitor phenotypic changes and detect cell stress...
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations



