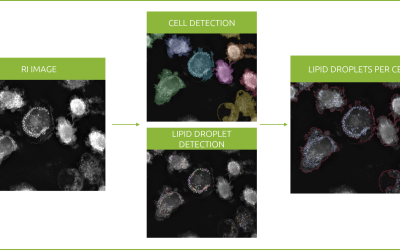
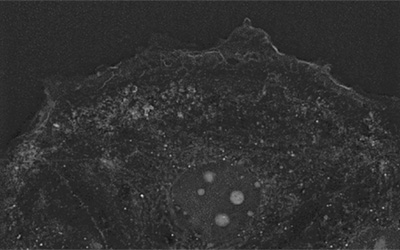
Tumor-associated macrophages secrete extracellular vesicles (EVs) that range from a variety of sizes and subcellular origins[1]–[5].
Exosomes (30-150nm) are a type of EVs originated from endosomal multivesicular bodies[4], [6]. Other types of EVs result from the shedding of plasma membrane, which leads to the formation of microvesicles (100-1000nm) during steady-state conditions or to apoptotic bodies (100-5000nm) during cell death[4], [6].
Tumor-derived EVs are known to play a role in a number of tumor progression processes, such as metastasis and chemotherapy resistance[7]–[9].
Cianciaruso et al. developed a method to analyze tumor-associated macrophages’ secreted EVs[5].
Following the proposed method[5], tumor-derived EVs were isolated from breast cancer cells. They were then harvested and labelled with a fluorescent lipid.
After that, the tumor-derived EVs were added to a mouse macrophage culture and their uptake was monitored for 6 hours under Nanolive’s 3D Cell Explorer-fluo. While data regarding refractive index was obtained every 10 seconds, the cadence of fluorescence acquisitions was increased in order to avoid fluorescence-induced cell perturbations that may had compromised the observed phenomenon.
As observed in the footage, fluorescence intensity increases over time as EVs uptake increases.
Strikingly, EVs structures are visible using Nanolive’s imaging. They appear as small, dotty, membrane-dense structures appearing and growing at the center of the badly resolved fluorescent signal. Thanks to the high temporal resolution, fine dynamics of such process can be extracted.
[1] S. Caruso and I. K. H. Poon, “Apoptotic cell-derived extracellular vesicles: More than just debris,” Frontiers in Immunology, vol. 9, no. JUN. Frontiers Media S.A., 28-Jun-2018.
[2] M. Colombo, G. Raposo, and C. Théry, “Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles,” Annu. Rev. Cell Dev. Biol., vol. 30, no. 1, pp. 255–289, Oct. 2014.
[3] M. Mathieu, L. Martin-Jaular, G. Lavieu, and C. Théry, “Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication,” Nature Cell Biology, vol. 21, no. 1. Nature Publishing Group, pp. 9–17, 01-Jan-2019.
[4] G. Van Niel, G. D’Angelo, and G. Raposo, “Shedding light on the cell biology of extracellular vesicles,” Nature Reviews Molecular Cell Biology, vol. 19, no. 4. Nature Publishing Group, pp. 213–228, 01-Apr-2018.
[5] C. Cianciaruso et al., “Molecular Profiling and Functional Analysis of Macrophage-Derived Tumor Extracellular Vesicles,” Cell Rep., vol. 27, no. 10, pp. 3062-3080.e11, Jun. 2019.
[6] M. Yáñez-Mó et al., “Biological properties of extracellular vesicles and their physiological functions,” Journal of Extracellular Vesicles, vol. 4, no. 2015. Co-Action Publishing, pp. 1–60, 2015.
[7] C. F. Ruivo, B. Arbara Adem, M. Silva, S. Onia, and A. Melo, “The Biology of Cancer Exosomes: Insights and New Perspectives,” 2017.
[8] I. Keklikoglou, C. Cianciaruso, E. Güç, … M. S.-N. cell, and undefined 2019, “Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models,” nature.com.
[9] A. Becker, B. K. Thakur, J. M. Weiss, H. S. Kim, H. Peinado, and D. Lyden, “Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis,” Cancer Cell, vol. 30, no. 6. Cell Press, pp. 836–848, 12-Dec-2016.
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations