What does fixation do to your cells’ morphology?
In life sciences, fixation refers to the process of preservation of histological, cytological and microbiological specimens such as cells and tissues at the status they were originally taken. A protocol of fixation usually precedes immunostaining, which is essential to immobilize biological structures and make them accessible to antibodies.
There are different mechanisms of fixation depending on the reagent used. As already shown in a previous publication from Sandoz et al (Fig.2A), dehydrating fixation methods severely affected organelles structure preservation and thereby their RI. A widely used alternative is paraformaldehyde (PFA), which acts creating covalent chemical cross-links between proteins. Even if PFA rapidly penetrates the sample, the process can take up to a few weeks in case of human biopsies for example.
Since fixation aims to provide a snapshot of a living situation, we visualized the process of fixing living samples with PFA using Nanolive’s 3D Cell Explorer. We conclude that cell structures are affected by the fixation process.
Two samples of living mouse pre-adipocytes were imaged for 30 min before initiating the fixation process. Images were taken every 15 seconds both before and after fixation. On the 0.2% PFA condition, PFA was directly diluted in medium with a final concentration of 0.2% PFA at 30min. On the 2% PFA condition, the medium was completely removed and replaced by 2% PFA in PBS after 30min. The process of fixation was recorded for up to 1 hour.
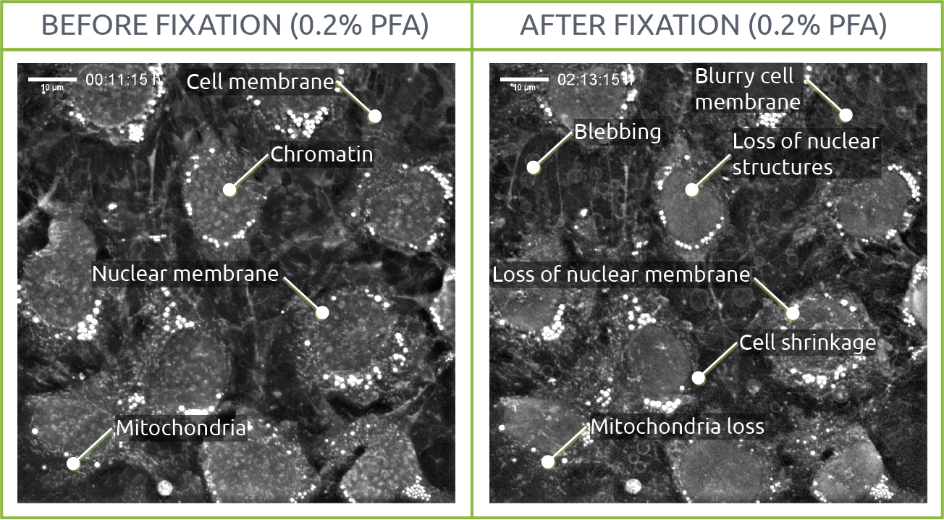
0.2% PFA
The effects of fixation could be observed early on at the level of cellular structures. In this sense, while chromatin, nuclear membrane and mitochondria were distinguishable before fixation, their shape and density were affected after PFA was added to the medium. This same phenomenon was observed on the cellular membrane, with cells shrinking. Several blebs appeared throughout the sample.
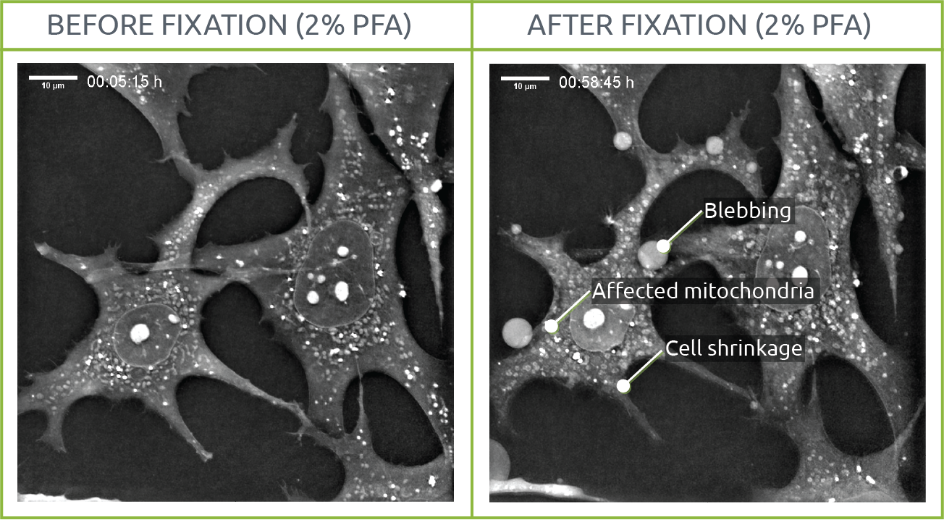
2% PFA
Morphological changes such as variations in mitochondrial shape and density or cell shrinkage were visualized. In a similar way to the cells observed on the 0.2% PFA, membrane degradation led to blebbing.
Interestingly, mitochondria shape evolved into almost perfect rounded shapes after fixation. A phenomenon of “granularity” was also observed in those organelles: while mitochondrial content lost density, their membrane looked “brighter” than before fixation probably due to fixation-derived changes in membrane nature and integrity.
Highlights
All in all, these observations strongly question the use of fixed samples in research involving membrane dependent systems.
A concentration as low as 0.2% PFA already induces very strong cellular changes at the molecular level, which are translated in an observation that does not meet the characteristics of living cells. These modifications are much more accentuated amongst membranes (mitochondrial, nuclear and plasma).
These conclusions correlate with previous research on the subject[1], [2]. PFA fixation has been shown to induce strong perturbations of cellular nanostructures[2], and inefficiently fix glycosylphosphatidylinositol-anchored, phospholipid-anchored or cholesterol-anchored membrane proteins, leading to observation of aberrant clustering of proteins observed by subsequent immune-staining[1].
[1] K. A. K. Tanaka et al., “Membrane molecules mobile even after chemical fixation,” Nature Methods, vol. 7, no. 11. pp. 865–866, Nov-2010.
[2] Y. Li et al., “The effects of chemical fixation on the cellular nanostructure,” Exp. Cell Res., vol. 358, no. 2, pp. 253–259, Sep. 2017.
