Superparamagnetic iron oxide nanoparticles (SPIONs) are widely used in biomedical applications
Twenty years ago, the idea of tiny magnetic nanoparticles flying around your body killing bad cells and healing others could well have been the blurb for the latest Pixar movie, but today, superparamagnetic iron oxide nanoparticles (SPIONs) are one of the hottest topics in medical research, playing a key role in magnetic hyperthermia (1), targeted drug delivery (2) and forming effective MRI contrast agents for cell labeling (3).
One of the reasons for the astronomical success of SPIONs is the ease at which their properties can be manipulated to suit the target tissue or cell type. Assessing the efficiency of modified particles requires techniques that are capable of quantifying both cellular particle load and discriminating between cell membrane-associated and intracellular particles (4).
Superparamagnetic iron oxide nanoparticles (SPIONs) in pancreatic cells
Superparamagnetic iron oxide nanoparticles (SPIONs) in pancreatic cells, imaged under Nanolive 3D Cell Explorer-fluo. Sequence: Z-projection of refractive index data, 3D reconstruction of refractive index data, and 3D reconstruction of refractive index data digitally stained
Could Nanolive’s label-free live cell imaging help?
When Ralf Meister donated a 3D Cell Explorer-fluo microscope to the Section of Experimental Oncology and Nanomedicine (SEON) at the University Hospital in Erlangen (Bavaria, Germany), Dr. Ralf Friedrich, Prof. Christoph Alexiou and colleagues sensed an opportunity: if Nanolive imaging could detect unlabeled SPIONs in living cells in 3D, this would overcome the need to fix cells (for phase contrast microscopy) or use fluorophore labels, which affect the physicochemical properties of SPION particles and can alter how they bind to cells (5).
So, the team set out to test this, incubating various pancreatic cell lines with lauric acid/albumin hybrid coated SPIONs, and quantifying content uptake using Nanolive imaging, fluorescence microscopy, flow cytometry, and microwave plasma-atomic emission spectrometry (MP-AES). The results published last week in the scientific journal Nanotechnology, Science and Applications showed that Nanolive imaging was able to quantify cell-specific differences in the incorporation of unlabeled SPIONs with an accuracy similar to MP-AES.
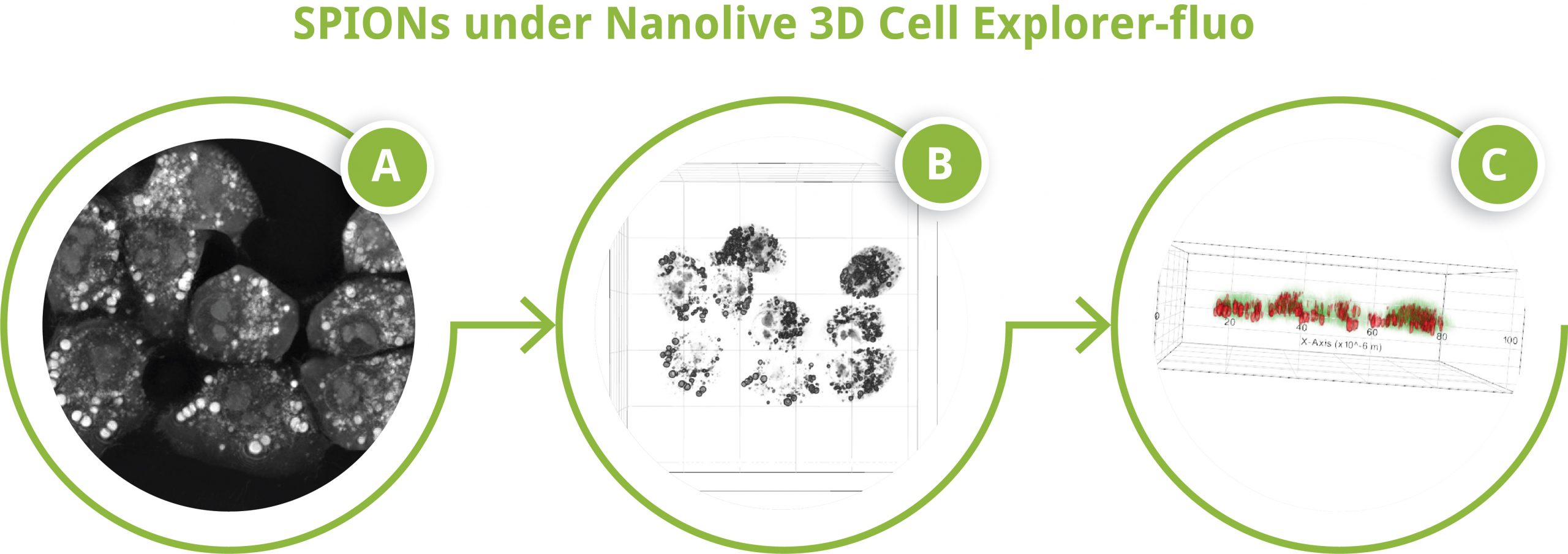
SPIONs under Nanolive 3D Cell Explorer-fluo
SPIONs under Nanolive 3D Cell Explorer-fluo. A) Z-projection of refractive index data, B) 3D reconstruction of refractive index data, C) 3D reconstruction of digitally stained refractive index data
The (highly promising) results
Nanolive imaging was deemed to be advantageous compared to fluorescence microscopy, which was judged to be “unsuitable” for assessing the performance of fluorescently unlabeled SPIONs, while it was recommended as being a complementary technique to flow cytometry, which the authors stressed remains “indispensable for a thorough characterization of particles’ impact upon cells”. The great advantage that Nanolive imaging offers over optical microscopy techniques though, is “the 3D representation of particle aggregates and the ability to distinguish between membrane-bound and incorporated particles”.
All in all, this is an extremely important proof-of-concept study that highlights the enormous promise and potential of Nanolive cell imaging for SPION development, investigation, and validation (4), and we would all like to extend our congratulations for such a thorough publication.
To read the full publication click here.
References
(1) Blanco-Andujar, C., Walter, A., Cotin, G., Bordeianu, C., Mertz, D., Felder-Flesch, D. and Begin-Colin, S., 2016. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine, 11(14):1889-1910.
(2) Tietze R, Zaloga J, Unterweger H, Lyer S, Friedrich RP, Janko C, Pöttler M, Dürr S, Alexiou C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015 Dec 18;468(3):463-70.
(3) Wei H, Bruns OT, Kaul MG, Hansen EC, Barch M, Wiśniowska A, Chen O, Chen Y, Li N, Okada S, Cordero JM. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA. 2017 Feb 28;114(9):2325-30.
(4) Friedrich RP, Schreiber E, Tietze R, Yang H, Pilarsky C, Alexiou C. Intracellular Quantification and localization of label-free iron oxide nanoparticles by holotomographic microscopy. Nanotechnol. Sci. Appl. 2020 Dec 9;13:119-30.
(5) Yin L, Wang W, Wang S, Zhang F, Zhang S, Tao N. How does fluorescent labeling affect the binding kinetics of proteins with intact cells? Biosens. Bioelectron. 2015;66:412–416.
Read our latest news
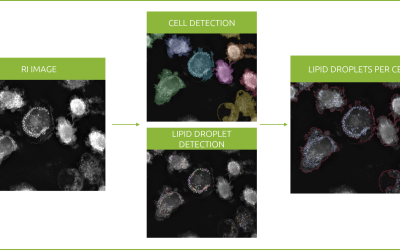
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations