Animal African trypanosomosis (AAT) is a severe threat to animal health
Animal African trypanosomosis (AAT), also known as Nagana has been identified by the World Health Organization (WHO) as one of the biggest threats to food security and economic development in sub-Saharan Africa (1). The devastating parasitic disease ravages cattle, sheep, goats, and horses, by reducing calving rate, meat, and milk production (2), and increasing mortality. The economic damage is estimated to be over US $4.75 billion each year (3).
AAT is caused by a flagellated euglenozoan protozoa with an unusual indirect life cycle
Trypanosoma brucei brucei, a single cell flagellate, is responsible for AAT, and has an interesting indirect life cycle, since it shuttles between an insect vector (tsetse flies) and a mammalian hosts (Fig. 1). In both phases of its life cycle, colonization of the host occurs extracellularly, e.g. in the bloodstream of the mammalian host, and includes the proliferation of rapidly dividing trypanosome forms before a resting (quiescent) state is achieved (Fig. 1). But how does the long, slender bloodstream form escape host cell immune responses during this phase?
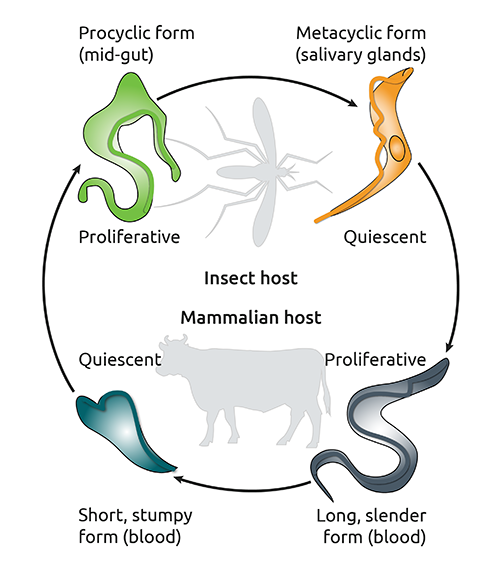
Fig. 1
The life cycle of Trypanosoma brucei brucei. Adapted from (4).
Trypanosomes induce polymorphonuclear neutrophil (PMN) activation as well as neutrophil extracellular traps (NETs) release in its host
Prof. Dr. Carlos Hermosilla and Prof. Dr. Anja Taubert from the Institute of Parasitology at the Justus Liebig University Giessen in Germany, used their 3D Cell Explorer-fluo to answer whether trypanosomes induce NET formation in bovine polymorphonuclear neutrophils (PMN). Last year their results were published in the scientific journal, Frontiers in Immunology (5). Co-author Dr. Iván Conejeros was kindly enough to send us the footage. The team confronted T. b. brucei trypomastigotes with bovine PMN and imaged their interactions for 3 h. Two fluorescent dyes were added to the co-culture; DRAQ5™; a cell permeable fluorescent dye that stains nucleic acids in living cells and SYTOX® Green; a nucleic acid stain that is impermeable to living cells and thus, can be used as a marker of extracellular DNA besides the normal use of dead cells staining. Refractive index (RI) images and fluorescence images (DAPI- and FITC channels) were acquired each minute.
Watch the process of NETosis in real time
Here, we observe the interactions between motile trypomastigotes and PMN in real time. The contact between both leads to modifications in the shape of the PMN nucleus. Usually, this is an expansion of the nuclear area, as is evidenced by the increase in the DRAQ5™ signal over time. By the end of the experiment, a SYTOX® Green signal appears in some cells, indicative of their cell death process. The overlap in the two signals suggests that extracellular DNA release is involved, a classical feature of the cell death process called NETosis (6). The presence and phenotypes of NETs were investigated further using scanning electron microscopy (SEM)-, confocal microscopy- and immunofluorescence microscopy analysis. Read the publication here.
This is not the first time that parasite-mediated NETs formation has been investigated using Nanolive technology. The same group reported a similar response of PMN to Besnoitia besnoiti bradyzoites – the quiescent parasite stages formed inside intracellular tissue cysts that are responsible for another bovine disease – besnoitiosis (7). Read this publication here.
References
[1] World Health Organization, Human African trypanosomiasis, viewed on April, 2021 <https://www.who.int/trypanosomiasis_african/parasite/en/>
[2] Swallow BM. Impacts of trypanosomiasis on African agriculture. PAAT Technical and Scientific Series (FAO). 2000
[3] Budd, L. DFID-funded tsetse and trypanosome research and development since 1980. Vol. 2. Economic analysis. Aylesford, UK, DFID Livestock Production, Animal Health and Natural Resources Systems Research Programmes. 123 pp. 1999
[4] Pays E, Vanhollebeke B, Vanhamme L, Paturiaux-Hanocq F, Nolan DP, Pérez-Morga D. The trypanolytic factor of human serum. Nature Reviews Microbiology. 4(6):477-86. 2006
[5] Grob D, Conejeros I, Velásquez ZD, Preußer C, Gärtner U, Alarcón P, Burgos RA, Hermosilla C, Taubert A. Trypanosoma brucei brucei Induces Polymorphonuclear Neutrophil Activation and Neutrophil Extracellular Traps Release. Frontiers in Immunology. 11, 559561. 2020
[6] Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science. 303, 1532–1535. 2004
[7] Zhou E, Silva LM, Conejeros I, Velásquez ZD, Hirz M, Gärtner U, Jacquiet P, Taubert A, Hermosilla C. Besnoitia besnoiti bradyzoite stages induce suicidal-and rapid vital-NETosis. Parasitology. 1;147(4):401-9. 2020
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging