What is Paclitaxel (Taxol) and how does it work?
Paclitaxel, perhaps better known by its brand name Taxol, has long been used as a chemotherapy drug and has been used to treat numerous types of cancer [1]. It is a cytoskeletal drug, which binds to a specific site on β-tubulin [2], a major component of the microtubules that form the structure and shape of eukaryotic cells.
Taxol works by stabilizing the microtubule polymer, preventing the disassembly of microtubules during the fourth phase of cell division, metaphase [3,4]. We have already written a detailed blogpost about the characteristic phases of mitosis but as a reminder, metaphase describes the stage when the cell’s chromosomes align along an invisible line in the center of the cell called the metaphasic plate. Spindle microtubules, which are attached to protein formations called kinetochores (formed earlier in mitosis), play a key role in this process; generating the forces needed to suspend the chromosomes along the metaphasic plate [5].
This is an important checkpoint in the cell cycle [6]. Only when all chromosomes are aligned, and every kinetochore is properly attached to a bundle of microtubules, can the cell proceed to enter anaphase. Taxol disrupts normal microtubule dynamics, activating the spindle checkpoint, which triggers mitotic arrest and/or cell death via apoptosis [3,4].
Sounds good right? Unfortunately, as with all anticancer drugs, Taxol is not 100% effective. Its efficiency depends on several factors including drug uptake and efflux levels [7], variations in cell tubulin content [8], and evasion of apoptosis [9].
Quantitative morphological analysis of Taxol-induced multinucleation
In this video, we observe one of the most well-known morphological side-effects of Taxol survival, multinucleation [10]. 3T3-derived pre-adipocyte cells were exposed to 1 μM of Taxol and imaged using the 3×3 gridscan mode (a field of view of 275 μm2) on Nanolive’s automated microscope the CX-A. Images were captured every 3 mins for 20 h. The sub-cellular resolution of the microscopes allows us to observe the consequences of mitotic catastrophe in spectacular detail while EVE Analytics, Nanolive’s complete software allows to quantify the increase in average cell dry mass density resulting from multinucleation.
What causes multinucleation?
Multinucleation is thought to be a result of chromosomal instability or telomere shortening [9], both of which can stimulate chromosome fusion.
Our experiment shows that this cell line at least, can tolerate multinucleation over short periods of time, which may prove useful for understanding of the properties of multinucleated cells, a subject of interest for researchers attempting to use cells cultured in vitro for tissue engineering [11].
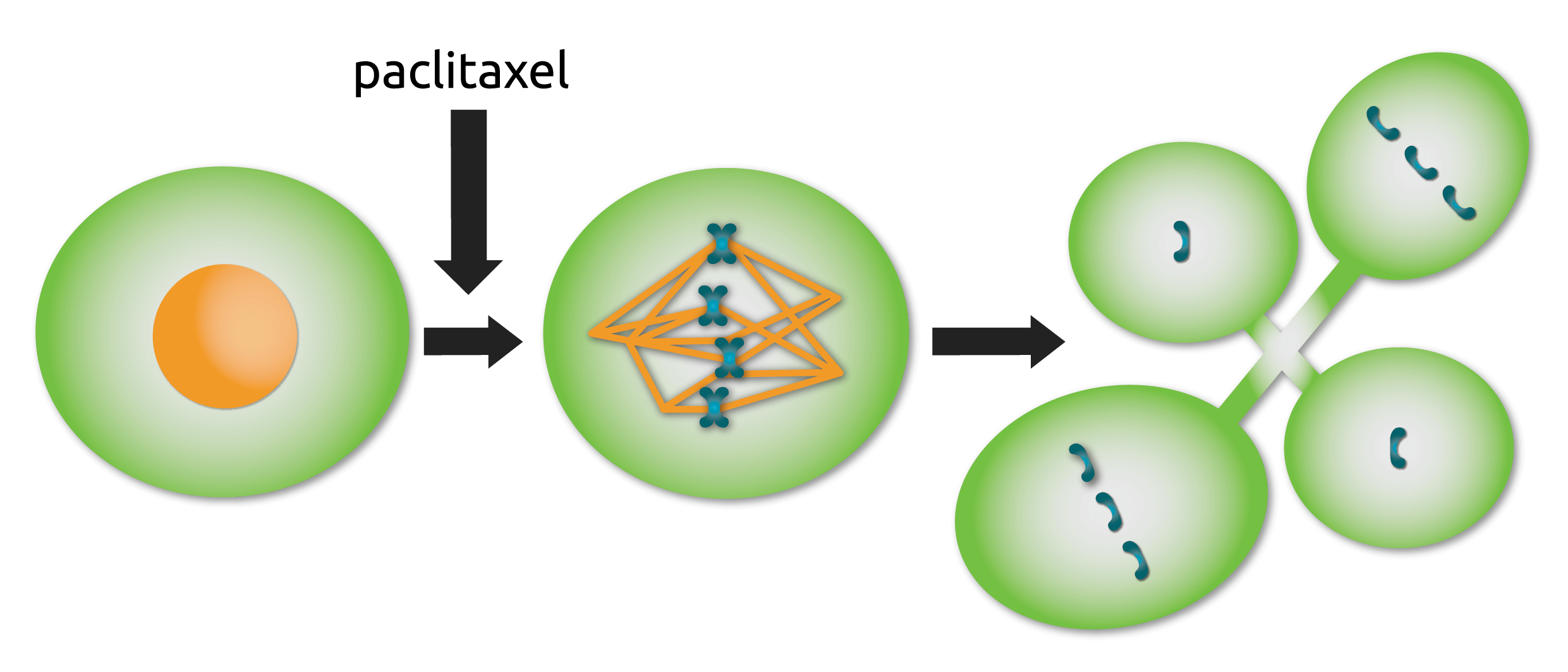
Fig. 1
Multinucleation following Taxol-addition. Cells entering mitosis in the presence of Taxol form abnormal spindles that contain additional spindle poles. Rather than mounting a long-term mitotic arrest, these cells enter anaphase and divide their chromosomes in multiple directions, resulting in a multi-nucleated cell. Adapted from [12].
References
[1] Barbuti A.M. and Chen Z.S. Cancers. 7(4): 2360-2371 (2015).
[2] Snyder J.P. et al. Proc. Natl. Acad. Sci. USA. 98(9): 5312-5316 (2001).
[3] Schiff P.B. et al. Nature. 277: 665-667 (1979).
[4] Schiff P.B. and Horwitz S.B. Proc. Natl. Acad. Sci. USA. 77: 1561-1565 (1980).
[5] Maiato H. et al. J. Cell Sci. 1;117(23): 5461-5477 (2004).
[6] Gorbsky G.J. Trends Cell Biol. 1;5(4): 143-148 (1995).
[7] Takano M. et al. Drug Metab. Pharmacokinet. 24: 418-427 (2009).
[8] Jaffrezou J.P. et al. Oncol. Res. 7: 517-527 (1995).
[9] Sumantran V.N. et al. Cancer Res. 55: 2507-2510 (1995).
[10] Park J.E. et al. Biochem. Biophys. Res. Commun. 14;404(2): 615-621 (2011).
[11] Sugita S. et al. Micromachines. 10(2): 156 (2019).
[12] Weaver BA. Mol. Biol. Cell. 15;25(18): 2677-2681 (2014).
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging