Cancer
A word pernicious enough to make your blood run cold. And one that 19.3 million people have the misfortune to hear every year. Cancer has the unenviable crown of being the leading cause of death worldwide, claiming more than 10 million lives in 2020 alone. According to the World Health Organisation (WHO), breast, lung, and colon and rectum cancers were the 3 most diagnosed cancers in 2020 (2.26, 2.1 and 1.93 million cases), while lung, colon and rectum, and liver cancer were responsible for the most deaths (1.80 million, 935,000 deaths, and 830,000 deaths). It is then, no surprise that cancer research is a multi-billion-dollar industry. So how much progress have we made in understanding and treating cancer? And how can we speed up cancer research in the future? In this blogpost, we examine the main eras that characterize cancer research (1) and predict the next epoch-making development.
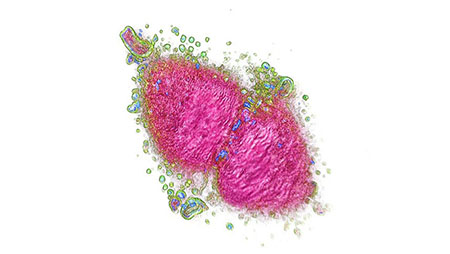
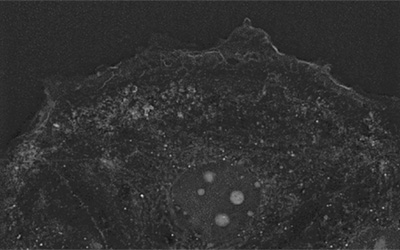
Image: Cancer cell imaged and digitally stained with the 3D Cell Explorer.
1895-1900. The discovery of X-rays and radioactivity.
Before 1895, surgical treatment was the only therapeutic option for treating cancer. The accidental discovery of X-rays in 1895 by German scientist Wilhelm Conrad Roentgen ushered in a new era in the treatment of cancer (2). Within a year X-rays were being used to treat breast cancer (3), and within three years Maria Sklodowska-Curie and her husband Pierre Curie discovered radium as a natural source of radioactivity (4).
1900-1940. The radiotherapy era.
Scientists quickly learned that exposure to high levels of radiation had very undesirable side effects (causing skin burns and sterility in the worst cases), so the early part of the 20th century was spent determining how X-rays and radium could best be used in medicine. One important development was the creation of the Coolidge tube in 1913 (5), which emits higher energy X-rays, allowing doctors to treat deeper cancers. Another breakthrough came in the early 1920s, when a Parisian scientist, Claudius Regaud discovered that radiotherapy works best when applied in multiple, small doses (radiation fractionation; (5)). In 1928, the International Commission on Radiological Protection Radiotherapy was formed, leading to its official recognition as a medical discipline. The safety and efficacy of radiotherapy has been vastly improved since it was developed and is still widely used for cancer treatment today.
1940-1970. The chemotherapy era.
Chemotherapy refers to the use of drugs to kill fast-growing or cancerous cells; the goal being to completely eradicate the disease before it can spread and metastasize in the body. German scientist Paul Ehrlich is largely credited as being the founder chemotherapy (6), but his seminal work on small molecules (e.g., Salvarsan) was so ground-breaking that it was largely unrecognized before the second World War. Identifying the potential benefits of chemotherapy had a deadly source: the use of mustard gas as a weapon. Millions of people were poisoned in what was surely one of the most horrific “advances” in modern warfare. But autopsies on the victims revealed something surprising; all had an extremely low white blood cell count. This led to the suggestion that chemical agents could be useful in killing cancer cells. A therapeutic trial administering nitrogen mustard in low doses followed with marked regression in cancer observed in patients suffering from lymphoma. These studies led to the synthesis and testing of several related new compounds, including chlorambucil and cyclophosphamide.
1970-2010. The targeted therapy era.
In 1977, Fred Sanger and his team at the Medical Research Council Laboratory of Molecular Biology in Cambridge (UK) made a scientific discovery that would change the face of medicine forever. They developed DNA sequencing; a way to read genetic code (7). Later, advances in bioinformatics would facilitate whole genome sequencing and lead to the launch of the Human Genome Project (HGP), an ambitious international research effort to determine the DNA sequence of the entire human genome. The HGP was launched in 1990 and successfully finished in 2003. Finally, researchers could attribute specific gene alterations to specific diseases. Cancer researchers study the structure of cancer genomes and identify alterations in the genes that drive and maintain tumorigenesis. This allows them to design and synthesize drugs that target specific genes or proteins. Targeted therapy is still a very active field in anticancer drug development (8) and covers all small selective inhibitory molecules and monoclonal antibodies, both synthetic and biologic.

2010-present. The immunotherapy era.
Cancer immunotherapy is currently the brightest beacon of light in the fight against cancer (9). This approach involves the artificial stimulation (activation or suppression) of the immune response in order to boost the body’s natural defense system against cancer. One of the ways cancer cells evade our natural immune defense is by sending T cells an “off” signal, preventing T cells from attacking cancerous cells. Immunotherapy drugs (typically monoclonal antibodies) called immune checkpoint inhibitors block the transmission of this signal, and so the T cell response remains active. Chimeric antigen receptor-T (CAR-T) therapy is another promising example of immunotherapy (10). In CAR-T therapy a patient’s cells are extracted and modified in the lab to add a receptor that is specific to an antigen expressed on tumor cells but not healthy cells. The modified T cells are then infused back into the patient thus acting as a “living drug” against cancer cells.
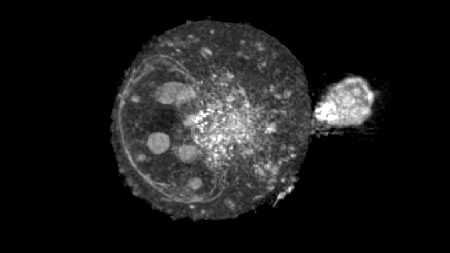
Image: T-Cell attacking a cancer cell – imaged with the 3D Cell Explorer.
2022-the future. Nanolive’s era?
One of the fundamental issues researchers currently face in the development of novel immunotherapeutics is the cost of bringing a drug from synthesis to production. A single drug is estimated to take 14 years (11) and costs more than US $3 billion to bring to market (12). One way of speeding up time and cutting costs is to optimize the pre-clinical phases of the drug discovery pipeline. Investing in optimal in vitro cell-based assays is encouraged as this reduces reliance on animal testing (13). In the past, cell-based assays were largely restricted to end-point techniques, which reveal whether cells are killed by a drug, but which fail to elucidate the dynamics or mechanisms behind the response of the cells. The development of inexpensive, label-free holotomographic microscopes has changed the game, offering researchers a non-invasive, unbiased view of a drug’s efficacy, over infinite amounts of time. Only one platform though currently provides truly quantitative, high content data and a means of quantifying the effect a drug has on T cell and cancer cell interactions in a label-free manner and that, we are proud to announce, is Nanolive. Our Live T Cell Assay is fully automated and outputs 5 unique quantitative metrics (number of T cells in contact with target cells, T cell area covered by target cells, target cell area covered by T cells, Mean T cell velocity, and average minimum distance between T cell and target cell), that will allow you to evaluate the capacity of your lead drug candidates to find, bind, stress, and kill cancer cells.
So, we are ready and equipped to join the fight, are you?
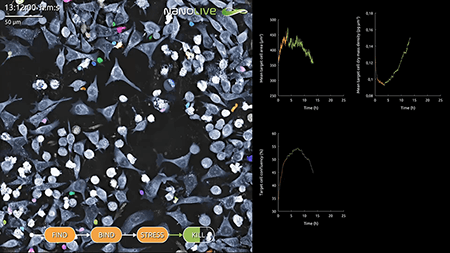
Image: Label-free AI-assisted segmentation of target cells and T cells in co-culture
References
(1) Falzone, L., et al. Front. Pharmacol. 13: 1300 (2018).
(2) Röntgen, W.C. Sitzungsberichte der physikalisch-medicinischen Gesellschaft zu Würzburg. 30: 132-141 (1895).
(3) Grubbe, E.H. Radiology; 21: 156-162 (1933).
(4) Becquerel, A.H., Curie, P. Compt. Rend. Acad. Sci. 132: 1289-1291 (1901).
(5) Lederman, M. Int. J. Radiat. Oncol. Biol. Phys. 7: 639-648 (1981).
(6) Kaufmann, S.H. Nat. Rev. Drug Discov. 7(5): 373-373 (2008).
(7) Sanger, F., et al. Proc. Natl. Acad. Sci. 1;74(12): 5463-7 (1977).
(8) Ke, X., Shen, L. 1;1(2): 69-75 (2017).
(9) Esfahani, K., et al. Curr. Oncol., 27: 87-97 (2020).
(10) Milone, M.C., et al. Nat. Cancer. 2(8): 780-793 (2021).
(11) Paul SM, et al. Nat. Rev. Drug Discov. 9: 203-14 (2010).
(12) Schuhmacher, A., et al. J. Transl. Med. 14(1): 1-1 (2016).
(13) Krewski, D., et al. J. Toxicol. Environ. Health B. Crit. Rev. 17; 13(2-4): 51-138 (2010).
Read our latest news
Revolutionizing lipid droplet analysis: insights from Nanolive’s Smart Lipid Droplet Assay Application Note
Introducing the Smart Lipid Droplet Assay: A breakthrough in label-free lipid droplet analysis Discover the power of Nanolive's Smart Lipid Droplet Assay (SLDA), the first smart digital assay to provide a push-button solution for analyzing lipid droplet dynamics,...
Food additives and gut health: new research from the University of Sydney
The team of Professor Wojciech Chrzanowski in the Sydney Pharmacy School at the University of Sydney have published their findings on the toxic effect of titanium nanoparticles found in food. The paper “Impact of nano-titanium dioxide extracted from food products on...
2023 scientific publications roundup
2023 has been a record year for clients using the Nanolive system in their scientific publications. The number of peer-reviewed publications has continued to increase, and there has been a real growth in groups publishing pre-prints to give a preview of their work....
Nanolive microscopes

CX-A
Automated live cell imaging: a unique walk-away solution for long-term live cell imaging of single cells and cell populations

3D CELL EXPLORER-fluo
Multimodal Complete Solution: combine high quality non-invasive 4D live cell imaging with fluorescence

3D CELL EXPLORER
Budget-friendly, easy-to-use, compact solution for high quality non-invasive 4D live cell imaging